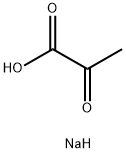
| Description |
Sodium pyruvate is the sodium salt of pyruvate. It is frequently supplemented to the cell culture medium to act as a source of energy since it is a key intermediate during the production of the high-energy ATP molecules inside cells. For example, it can be used as a carbon source for bacteria. It may also protect cell against hydrogen peroxide, an oxidant to scavenger oxygen radicals. It is an important metabolic intermediate in many essential metabolic pathways such as carbohydrate metabolism. For example, it is converted into acetyl coenzyme A and enters into the TCA cycle (Kreb’s cycle) in organisms. It is also involved in the amino acid metabolism in organisms. |
| Chemical Properties |
White to slightly yellow crystalline pow |
| Uses |
Intermediate in sugar metabolism and in enzymatic carbohydrate degradation (alcoholic fermentation) where it is converted to acetaldehyde and CO2 by carboxylase. In muscle, Pyruvic acid (derived from glycogen) is reduced to lactic acid during exertion, which is reoxidized and partially retransformed to glycogen during rest. The liver can convert Pyruvic acid to alanine by amination. A diagnostic agent for Parkinson disease |
| Biological Functions |
Sodium pyruvate (α-Ketopropionic acid sodium salt; 2-Oxopropanoic acid sodium salt;Pyruvic acid sodium; C3H3NaO3) as an important endogenous small molecules participates various tissue and organ metabolism processes that is the final product of glycolysis and the starting substrate for the tricarboxylic acid (TCA) cycle, and possesses antioxidant and scavenging free radical effects, thus widely using as buffer, excipient and antioxidant in medicine, diagnostic reagent and medical device.
Sodium pyruvate is an endogenous antioxidant and reactive oxygen radical scavenger. In this process, H2O2 or other reactive oxygen radicals are scavenged by sodium pyruvate by a nonenzymatic reaction or an oxidative dephosphorylation, and produce acetate, water and carbon dioxide. Thus, sodium pyruvate can suppress renal cellular injury induced by H2O2, such as lipid peroxidation of rat kidney homogenate, and cytosolic 51Cr release (a marker of cellular injury) from renal epithelial cells induced by H2O2. Thus, sodium pyruvate as effect antioxidant has potential in the clinical medication. Moreover, as effect antioxidant, sodium pyruvate is also as additives used in a vast range of foods and toiletries. |
| References |
https://en.wikipedia.org/wiki/Sodium_pyruvate
Taidi, Behnam, et al. "Effect of carbon source and concentration on the molecular mass of poly (3-hydroxybutyrate) produced by Methylobacterium extorquens and Alcaligenes eutrophus." Applied microbiology and biotechnology 40.6 (1994): 786-790.
https://www.alfa.com/zh-cn/catalog/A11148/
http://bio.lonza.com/uploads/tx_mwaxmarketingmaterial/Lonza_BenchGuides_Sodium_Pyruvate_Solution_100mM.pdf |